ATLANTA, GA — December 1, 2025 — Today, researchers, in partnership with HepVu, the Hepatitis B Foundation, and the National Viral Hepatitis Roundtable, released new data highlighting the significant, measurable health and economic consequences of delaying the infant hepatitis B (HepB) birth dose vaccine in the United States. The findings—based on a model of 2024 U.S. births—show that even short delays in vaccination lead to substantially more infections, severe long-term health complications, and sharply increased healthcare spending.
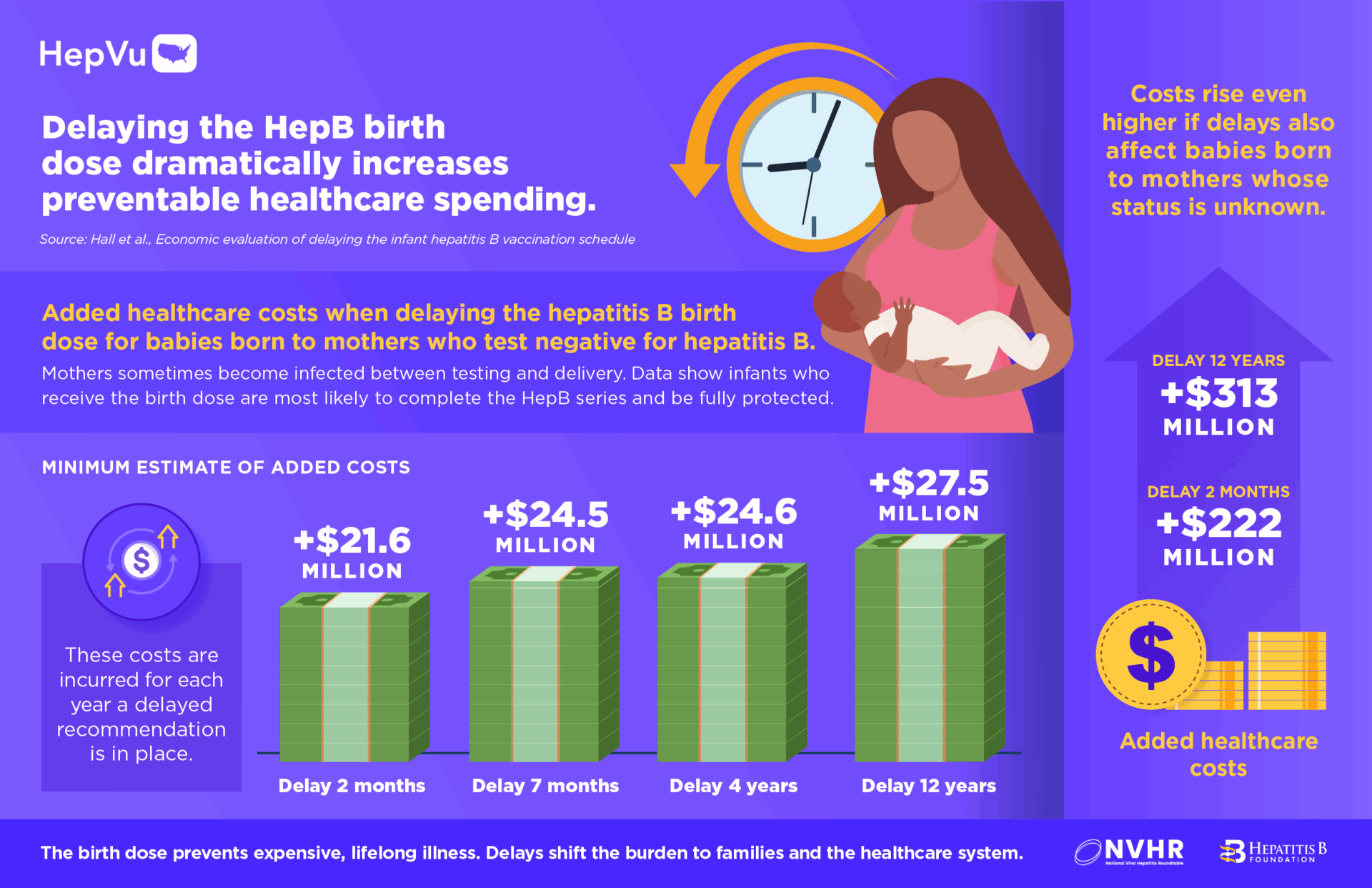
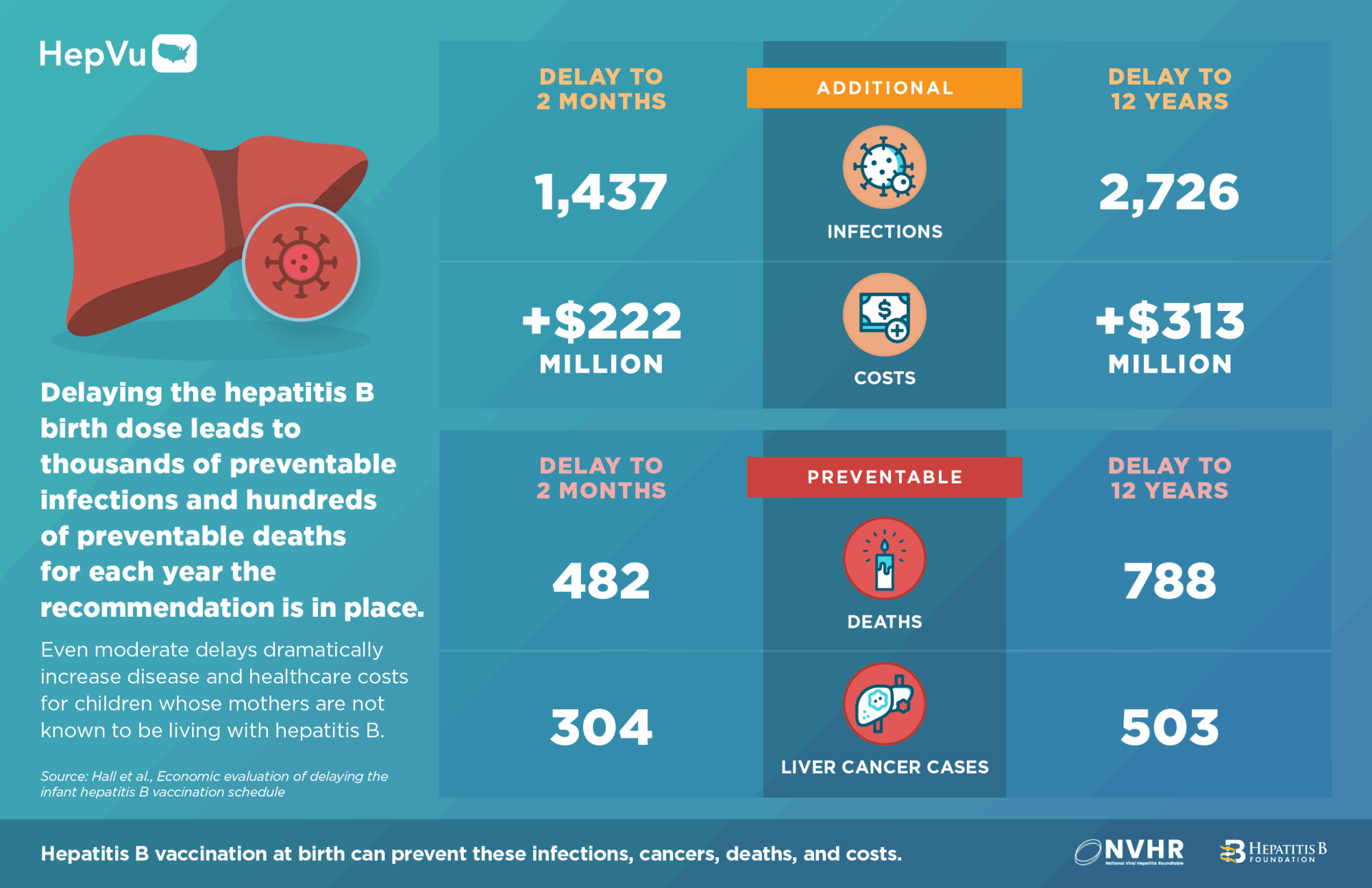
- By delaying the birth dose to 2 months among infants whose mothers are not known to be living with hepatitis B, there could be at least 1,400 preventable hepatitis B infections among children, 300 excess cases of liver cancer, 480 preventable deaths and over $222 million in excess healthcare costs, for each year the revised recommendation is in place.
- If the birth dose was delayed to 12 years, this would balloon to at least 2,700 preventable hepatitis B infections and $313 million in excess healthcare costs for each year the revised recommendation is in place.
“Our analysis makes clear that the hepatitis B birth dose is a critical tool for preventing infections that can last a lifetime,” said Eric Hall, PhD, lead author, Oregon Health & Science University – Portland State University School of Public Health. “When vaccination is delayed, whether by months or by years, we see predictable and preventable increases in new infections, chronic disease, liver cancer, and related deaths; these findings show how important timely protection at birth is for safeguarding children’s long-term health.”
The work was led by researchers and public health practitioners from Oregon Health & Science University, the Los Angeles County Department of Public Health, Emory University, and Cornell University. The model and results were released as a pre-print in medRxiv titled, Economic evaluation of delaying the infant hepatitis B vaccination schedule. This new analysis quantifies the impact of delaying the first HepB vaccination by months or years among infants whose mothers are not known to be living with hepatitis B. The authors have previously developed models on hepatitis B vaccination policies, within the framework outlined by the Advisory Committee on Immunization Practices (ACIP) Guidance for Health Economics Studies, which have informed prior ACIP decisions.
“These data provide a powerful, evidence-based foundation for decision-makers, health departments, and advocates who are working to strengthen infant immunization policies,” said Michaela Jackson, Program Director, Prevention Policy, Hepatitis B Foundation, “By quantifying the real-world consequences of delaying the hepatitis B birth dose, including thousands of preventable infections and hundreds of millions in avoidable healthcare costs, this analysis offers a clear, data-driven tool to inform policy discussions and ensure that all infants receive the timely protection they need.
“It also underscores that infant protection is strengthened when birth dose vaccination and maternal screening are used together as complementary components of one prevention strategy,” added Daniel Raymond, Director of Policy, National Viral Hepatitis Roundtable. “These findings should serve as a stark reminder as CDC’s Advisory Committee on Immunization Practices (ACIP) contemplates changes to longstanding childhood immunization recommendations: delaying HepB birth dose immunization jeopardizes the health of our children and three decades of progress towards hepatitis B elimination.”
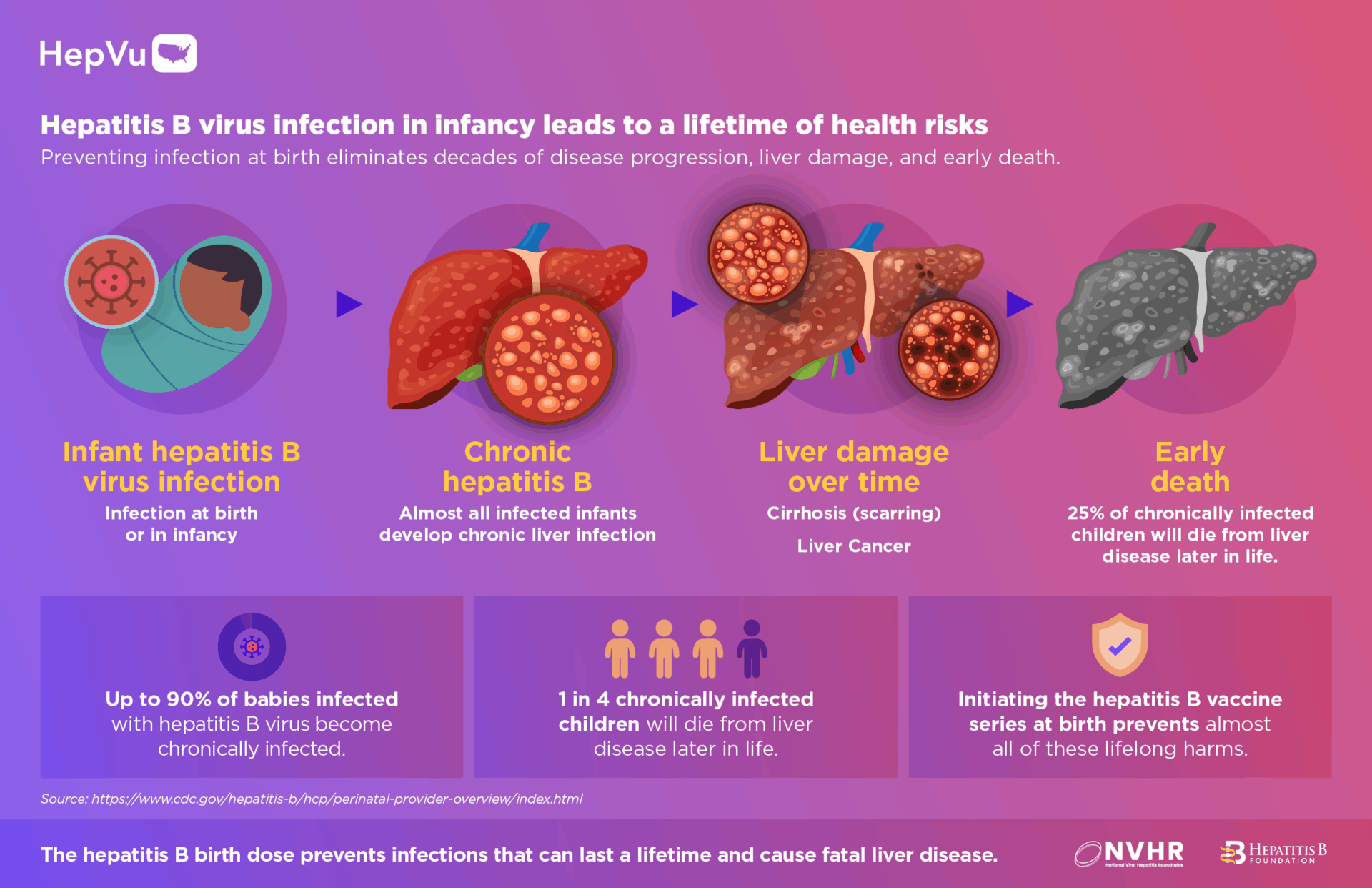
According to CDC’s vaccine safety recommendations, in a 4-year case series review of HepB vaccine reports among newborns, there were no serious health problems linked to the HepB vaccine. Decades of evidence demonstrate the HepB vaccine is safe and highly effective for preventing infection in childhood and later in life. Additionally, previous data show that infants who receive the first HepB vaccine dose at birth are more likely than those who do not receive the birth dose to complete the series and be fully protected from the virus.
Key Findings
Delays Increase Preventable Hepatitis B Infections
Modeling shows that for each year the delayed birth dose policy is in place for infants whose mothers test negative for hepatitis B
- Delaying the birth dose to 2 months results in at least 238 additional infections among children.
- Delaying to 7 months results in at least 438 additional infections.
- Delaying to 4 years results in at least 701 additional infections.
- Delaying to 12 years results in at least 740 additional infections.
These data show that providing the birth dose for infants whose mothers test negative during pregnancy is critical. Mothers sometimes become infected between hepatitis B testing and delivery.
If the birth dose was also delayed for infants of mothers with unknown hepatitis B status, there would be at least 1,437 (2-month delay) to 2,726 (12-year delay) additional infections for each year the delayed birth dose policy is in place.
Delays Lead to More Preventable Deaths and Liver Cancer
Delays also increase chronic hepatitis B infections, cirrhosis, liver cancer, and hepatitis B-related deaths, with the most pronounced effects to infants whose mothers were not tested during pregnancy.
Infants whose mothers test negative for hepatitis B experience substantial increases in severe outcomes. For each year a delayed birth dose policy is in place, modeling projects:
- Delaying the birth dose to 2 months results in at least 62 preventable deaths and 39 cases of liver cancer.
- Delaying to 12 years results in at least 163 preventable deaths and 107 cases of liver cancer.
When delays also apply to infants of mothers with unknown hepatitis B status, the impact is far greater— delaying the birth dose to 12 years results in at least 788 preventable deaths and 503 cases of liver cancer for each year the revised recommendation is in place.
Delays Dramatically Increase Healthcare Costs
Every delay scenario sharply increases healthcare spending due to new infections and lifelong management of chronic hepatitis B infection and liver disease:
For each year the delayed birth dose policy is in place for infants whose mothers test negative for hepatitis B:
- Delaying the birth dose to 2 months results in at least +$21.6 million in healthcare costs
- Delaying to 7 months results in at least +$24.5 million
- Delaying to 4 years results in at least +$24.6 million
- Delaying to 12 years results in at least +$27.5 million
When delays apply to infants whose mothers test negative and whose hepatitis B status is unknown:
- Each year the delayed birth dose policy remains in effect is expected to result in at least $222 million to $313 million in excess healthcare costs, depending on timing of the delay (2 months – 12 years).
- If the policy remains in effect for a decade, estimated excess healthcare costs total at least $2.22 billion–$3.13 billion. If pre-vaccination screening is required for older children prior to vaccination, total estimated costs rise substantially higher ($3.24 billion – $4.09 billion).
Delayed Protection Leaves Infants Exposed During Their Highest-Risk Period
- Children face the highest likelihood of developing chronic, lifelong infection if exposed to hepatitis B at birth. Up to 90% of infants infected in their first year of life become chronically infected, placing them at a significantly increased risk for developing liver cancer in their lifetime.
- 1 in 4 chronically infected children later die from liver disease.
The HepB birth dose prevents these infections and associated lifelong harms by providing immediate protection from transmission at birth and in childhood.
New Infographics Now Available on HepVu.org
To support public health practitioners, policymakers, and journalists, HepVu has published a series of visual summaries. All infographics can be republished with attribution to HepVu.
About HepVu
HepVu is a data-visualization platform presented as a partnership between Emory University and Gilead Science that is working to advance understanding of viral hepatitis in the United States. By presenting accessible, actionable data, HepVu supports policymakers, public health departments, researchers, and the public in efforts to reduce the burden of viral hepatitis nationwide.
About Hepatitis B Foundation
As the world’s leading hepatitis B advocacy and research organization, the Hepatitis B Foundation is one of the most active proponents of improving hepatitis B screening, prevention, and treatment of the disease. The Foundation is the only nonprofit organization solely dedicated to finding a cure for hepatitis B and improving the quality of life for those affected worldwide through research, education and patient advocacy. Founded in 1991, the Hepatitis B Foundation is based in Doylestown, Pa., with staff in Washington, D.C., and Philadelphia.
About National Viral Hepatitis Roundtable
The National Viral Hepatitis Roundtable (NVHR), a program of Hepatitis Education Project (HEP), brings patients, providers, public health leaders, and community partners together to advance viral hepatitis elimination in the United States.